CYP2C19 Pharmacogenomic Panel
Why Genotyping Matters

Metabolic Variability
PPIs are cleared mainly by CYP2C19; genotype can alter drug exposure >20-fold between ultra-rapid and poor metabolizers.
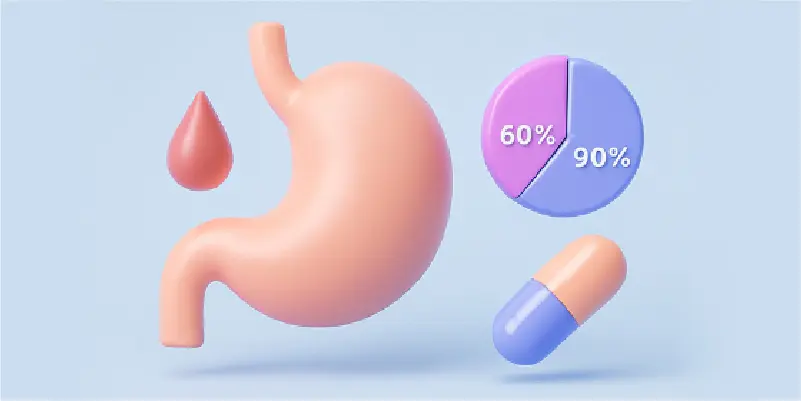
Therapeutic Impact
H. pylori eradication and erosive-esophagitis healing rates fall to ≤ 60% in rapid/ultra-rapid metabolizers (RM/UM) at standard doses, but exceed 90% in poor metabolizers (PM).

Guideline Consensus
The 2020 CPIC guideline provides explicit dose or drug-selection changes for every CYP2C19 phenotype; recommendations are graded strong or moderate.
Allele Prevalence
| Population | *2 (LOF) | *3 (LOF) | *17 (GOF) | % IM + PM | % RM + UM |
|---|---|---|---|---|---|
| East Asian | 29-35 % | 6-10 % | ≈ 3 % | ~45 % | ~4 % |
| European | 15 % | < 1 % | 20-25 % | ~18 % | ~35 % |
| African-American | 15 % | < 1 % | 16 % | ~18 % | ~30 % |
Who Should Be Tested?
| Patients starting long-term PPI therapy (GERD, Barrett’s, chronic NSAID prophylaxis) Prevent sub- or supra-therapeutic exposure |
|
| H. pylori eradication or erosive esophagitis requiring maximal acid suppression Higher cure rates when dose tailored |
|
| Refractory heartburn or PPI failure at standard dose RM/UM most likely |
|
| Infants/children needing prolonged PPI (e.g., eosinophilic esophagitis) Evidence of phenotype-driven dose differences |
|
| Individuals with East-Asian ancestry (high LOF allele rate) Nearly half are IM/PM |
Your Journey with Genolife Services
A clear path to optimizing Irinotecan therapy.
 |
Pre-test CounsellingUnderstand benefits, limitations, inheritance, and therapy options. |
 |
Sample Collection3 mL EDTA blood. |
 |
Expert InterpretationClinical Geneticist, Genetic Counsellor, Pharmacogenomic Pharmacologist, and Gastroenterologist review. |
 |
Actionable Report & Dose Planning |
Ready to plan for a safer health future?
Contact us for a consultation with our Genetic Counselor/Pharmacogenomics Specialist about the Proton Pump Inhibitors (PPIs) or other genetic tests.
References
- Lima JJ et al. CPIC Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin Pharmacol Ther. 2020.
- CPIC Supplement Tables (Phenotype & Dosing). 2020.
- Ionova Y et al. CYP2C19 *2/ 3/17 allele frequencies in 2.2 million individuals. Clin Transl Sci. 2020.
- Scott SA. CYP2C19*17 pharmacogene review. Pharmacogenomics J. 2012.
- CPIC Guideline PDF – exposure, efficacy & adverse effects discussion.







