UGT1A1 Pharmacogenomic Testing
Why Genotyping Changes Outcomes
Understanding the genetic link to Irinotecan toxicity.
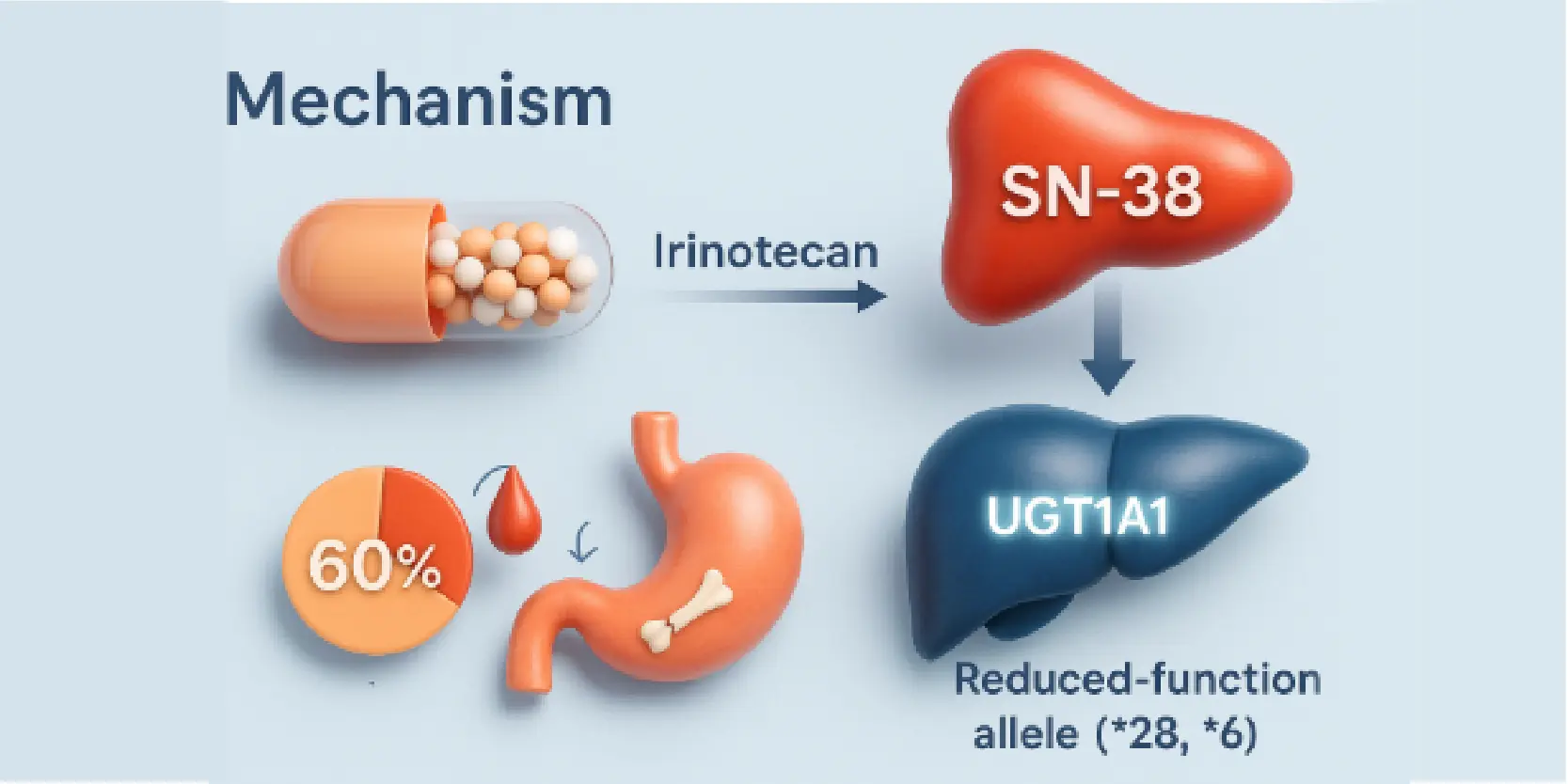
Mechanism of Toxicity
Irinotecan’s active metabolite (SN-38) is inactivated by UGT1A1. Reduced-function alleles (*28, *6) slow this process, increasing SN-38 exposure and the risk of severe side effects.
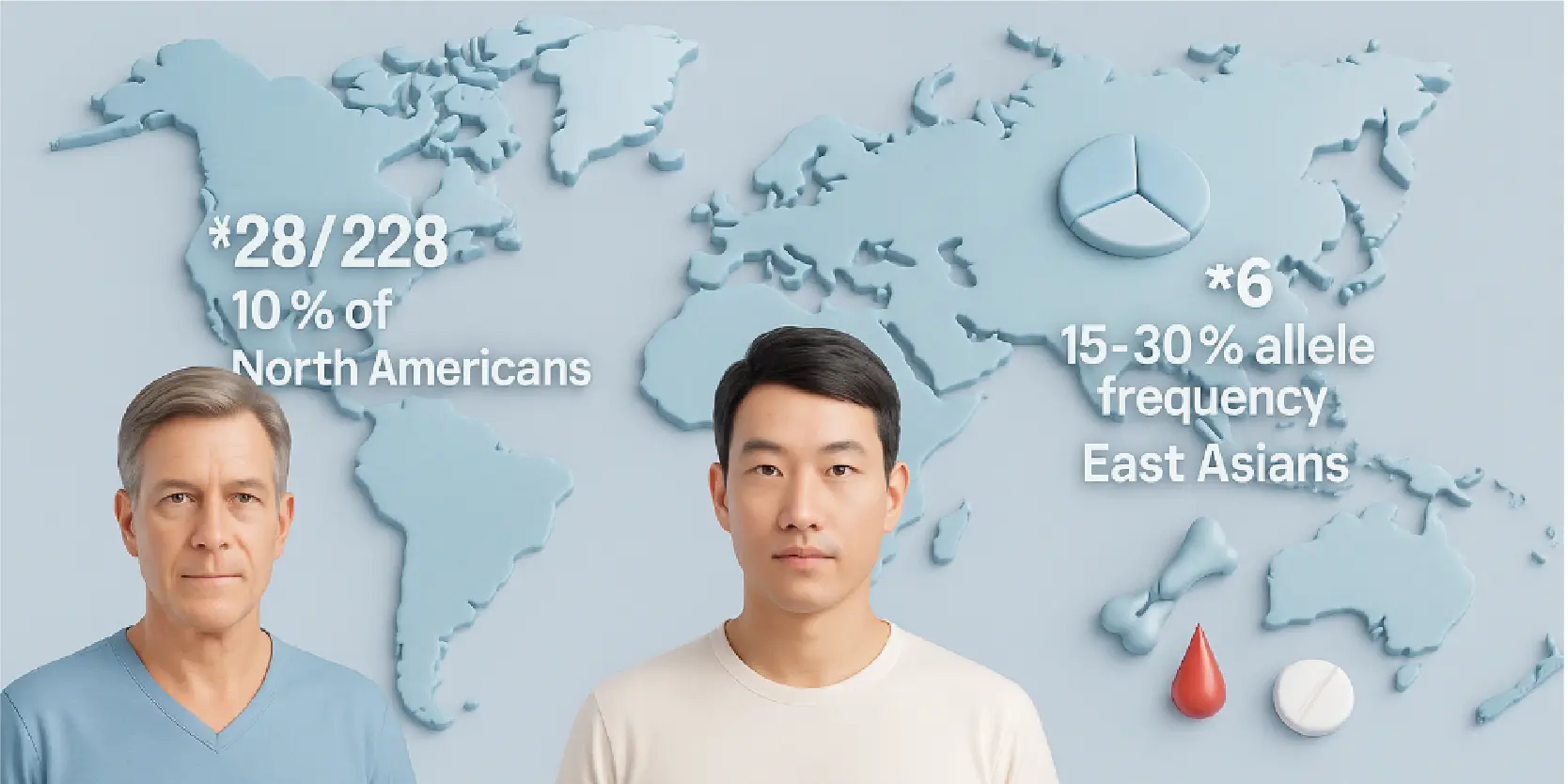
Who’s at Risk?
About 10% of North Americans are *28/*28 homozygotes. The *6 allele is common in East Asians (15-30%), predicting higher toxicity risk in these groups.
How Common Are the Risk Alleles?
| UGT1A1*28 allele: | ~0.26–0.31 in Caucasians; ~0.42–0.56 in African Americans; ~0.09–0.16 in Asians. |
| UGT1A1*6 allele: | ~0.15–0.30 in Chinese, Korean, and Japanese populations. |
Who Should Be Tested?
Identifying ideal candidates for pharmacogenomic testing.
- Patients starting irinotecan at ≥180 mg/m² or as single-agent – Highest genotype–toxicity signal; informs initial dosing
Your Journey with Genolife Services
A clear path to optimizing Irinotecan therapy.
 |
Pre-test CounsellingDiscuss benefits, limits, and alternative irinotecan dosing strategies. |
 |
Sample CollectionA simple blood sample is all that’s needed (no fasting required). |
 |
Expert InterpretationResults are reviewed by a team including a Clinical Geneticist, Pharmacist, and Oncologist. |
 |
Post testOffered for relatives who may also need irinotecan or have a history of related toxicity. |
Ready to plan for a safer health future?
Contact us for a consultation with our Genetic Counselor/Pharmacogenomics Specialist about the Irinotecan Toxicity Risk Panel or other genetic tests.
References
- NCBI Medical Genetics Summaries. Irinotecan Therapy and UGT1A1 Genotype. Updated overview of mechanism, allele frequencies, FDA labeling, and dose-toxicity relationships.
- PharmGKB. Irinotecan + UGT1A1 clinical and variant annotations; allele frequency resources.
- CPIC. UGT1A1–Irinotecan evidence status update (workstream). Confirms active evaluation of dosing guidance within CPIC







