Questions: What is the type of Moderna COVID-19 vaccine?
Answer: Moderna COVID-19 vaccine is mRNA vaccine (mRNA-1273) which is a novel vaccine protecting against COVID-19. This vaccine has been developed by ModernaTX, Inc. – an American pharmaceutical and biotechnology company. Messenger RNA (mRNA) vaccine gives instructions for our cells to make a harmless piece of viral spike glycoprotein. Once this genetic material gets into human cells, it uses our cells’ protein factories to produce the antigen that subsequently triggers an immune response to fight against the disease. This new COVID-19 vaccines using mRNA technology appears to be more than 90% effective and it is regarded as one of the most effective COVID-19 vaccines that are currently available.
Questions: How safe is Moderna COVID-19 vaccine?
Answer: Moderna COVID-19 vaccine was approved for its efficacy and safety by The European Medicines Agency or EMA. Moreover, Strategic Advisory Group of Experts on Immunization (SAGE), the principal advisory group to the World Health Organization (WHO) granted the use of Moderna COVID-19 vaccine.
- After the EMA reviewed both efficacy and safety of Moderna COVID-19 vaccine, the approval issued by the EMA has been imposed across the European Union. In addition, apart from European countries, Moderna COVID-19 vaccine has been widely used in many countries, such as the US, Canada, Japan and Israel.
- Although this vaccine has reported a high degree of safety, a 15-minute observation after vaccine administration is needed in order to monitor any allergic reactions or adverse reactions caused by the vaccine or any vaccine ingredients. In case that severe allergy or anaphylactic reaction develops after the first dose of Moderna COVID-19 vaccine, the second dose of Moderna COVID-19 vaccine must definitely be avoided.
- More importantly, long-term clinical studies are essential for monitoring the effectiveness and safety, including side effects or adverse reactions that have not been reported in short-term trials.
Questions: How is Moderna COVID-19 vaccine administered?
Answer: Moderna COVID-19 vaccine is administered intramuscularly as a series of two doses (0.5 mL/ dose) with a 4-week interval.
Questions: Who can be vaccinated with Moderna COVID-19 vaccine?
Answer: Moderna COVID-19 vaccine is currently authorized for use in people aged 12 and older. Moderna COVID-19 vaccine is recommended for:
- Patients with underlying diseases who are at greater risks of severe illness caused by COVID-19, e.g. diabetes, hypertension, asthma, lung and respiratory disease, liver disease and kidney disease.
- Healthcare workers who have close contact with suspected or confirmed COVID-19 cases.
- Patients with HIV infections.
- Breastfeeding women
For pregnant women, elderly people and patients who are immunocompromized, it is highly recommended to talk to your doctor prior to vaccinating whether the benefits of this vaccine outweigh the potential risks.
Questions: What are contraindications to Moderna COVID-19 vaccine?
Answer: Contraindications to Moderna COVID-19 vaccine include:
- People aged below 12.
- People who develop anaphylactic reactions or allergies instantly after receiving mRNA vaccine (any manufacturers) regardless of severity that might vary from skin rash, urticaria, swelling eye or face, chest discomfort and breathing difficulties.
- People who have any allergic reactions after receiving the first dose of Moderna COVID-19 vaccine.
- People who are allergic to polyethylene glycol (PEG) which is one of the ingredients in Moderna COVID-19 vaccine.
- People who are allergic to polysorbate. Since PEG and polysorbate are closely related to each other. If people are allergic to polysorbate, although it is not an ingredient of Moderna COVID-19 vaccine, they should NOT be vaccinated with Moderna COVID-19 vaccine.
Questions: What are common side effects caused by Moderna COVID-19 vaccine?
Answer: Common side effects of Moderna COVID-19 vaccine are:
- Pain, redness or swelling at the injection site.
- Fever or chills
- Tiredness
- Headache
- Nausea
These side effects usually happen within a day or two after getting the vaccine. They are normal signs that your body is building up protection. These symptoms should subside and go away within a few days. In case of pain or fever, paracetamol can be taken as advised. However, anaphylaxis and serious adverse reactions, including myocarditis (an inflammation of the heart muscle or myocardium) and pericarditis (an inflammation of the pericardium, a sac-like structure that surrounds the heart) have been rarely reported.
Questions: Can people infected with COVID-19 be vaccinated with Moderna COVID-19 vaccine?
Answer: For COVID-19 patients who have been treated with monoclonal antibodies or convalescent plasma therapy in the hospital, Moderna COVID-19 vaccine administration can be considered at least 90 days after COVID-19 treatment. For COVID-19 patients who did not receive monoclonal antibodies or convalescent plasma therapy, Moderna COVID-19 vaccine can be administered once SARS-CoV-2 RT-PCT test shows negative result (not detected) for at least 1 month. In this case, only single dose of Moderna COVID-19 vaccine is required.
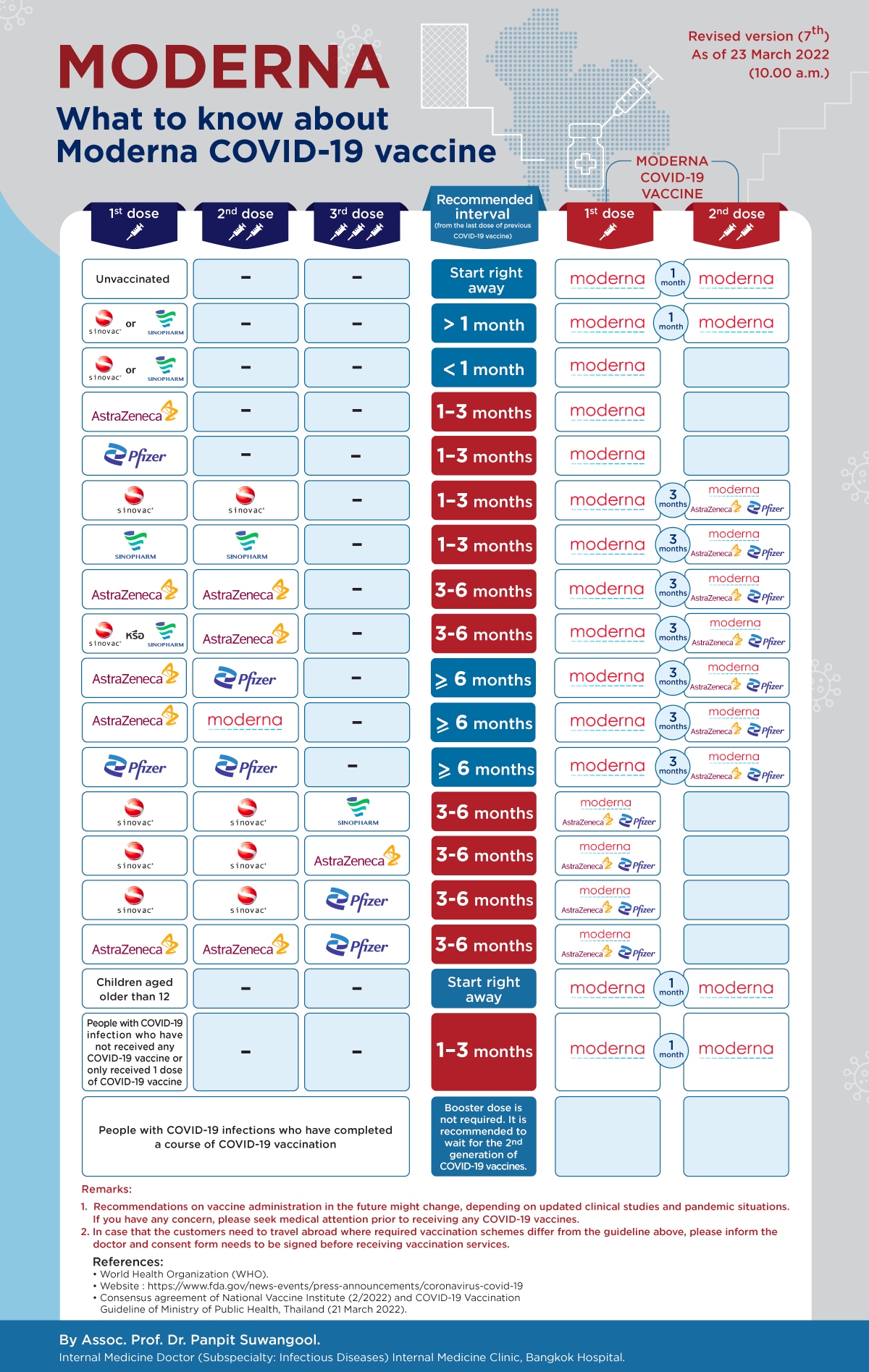
Questions: Can Moderna COVID-19 vaccine be used as a second shot interchangeably with other COVID-19 vaccines?
Answer: Due to a lack of firm clinical evidence in efficacy and safety, COVID-19 vaccines should not be used interchangeably. The first dose and second dose should ideally be the same vaccine. Nevertheless, if administrative guideline for vaccine interchangeability becomes conclusive, the recommendations might be changed in accordance with standard guideline.
Questions: Can Moderna COVID-19 vaccine be used as a booster dose after 2 doses of Sinovac or Sinopharm COVID-19 vaccine were fully given?
Answer: In case that 2 doses of Sinovac or Sinopharm COVID-19 vaccine were given: Moderna COVID-19 vaccine should be administered 1-3 months apart from the second dose of Sinovac or Sinopharm COVID-19 vaccine. Only one dose of Moderna COVID-19 vaccine is required after completing two doses of Sinovac or Sinopharm COVID-19 vaccine.
Questions: Can Moderna COVID-19 vaccine be used as a booster dose after 2 doses of AstraZeneca COVID-19 vaccine were fully given?
Answer: Moderna COVID-19 can be administered as a booster (third) dose 3-6 months after the second dose of AstraZeneca COVID-19 vaccine was given. However, only one dose of Moderna COVID-19 vaccine is required after completing two doses of AstraZeneca COVID-19 vaccine.
*** Update : 23 March 2022